Unpacking the Plumpudding Model of the Atom: How J.J. Thomson’s Revolutionary Idea Shaped Modern Atomic Science
- Introduction: The Birth of the Plumpudding Model
- J.J. Thomson and the Discovery of the Electron
- Core Concepts: Structure and Features of the Plumpudding Model
- Scientific Impact: How the Model Challenged Previous Atomic Theories
- Experimental Evidence: Support and Criticisms
- The Fall of the Plumpudding Model: Rutherford’s Gold Foil Experiment
- Legacy and Influence on Modern Atomic Theory
- Conclusion: Lessons from the Plumpudding Model
- Sources & References
Introduction: The Birth of the Plumpudding Model
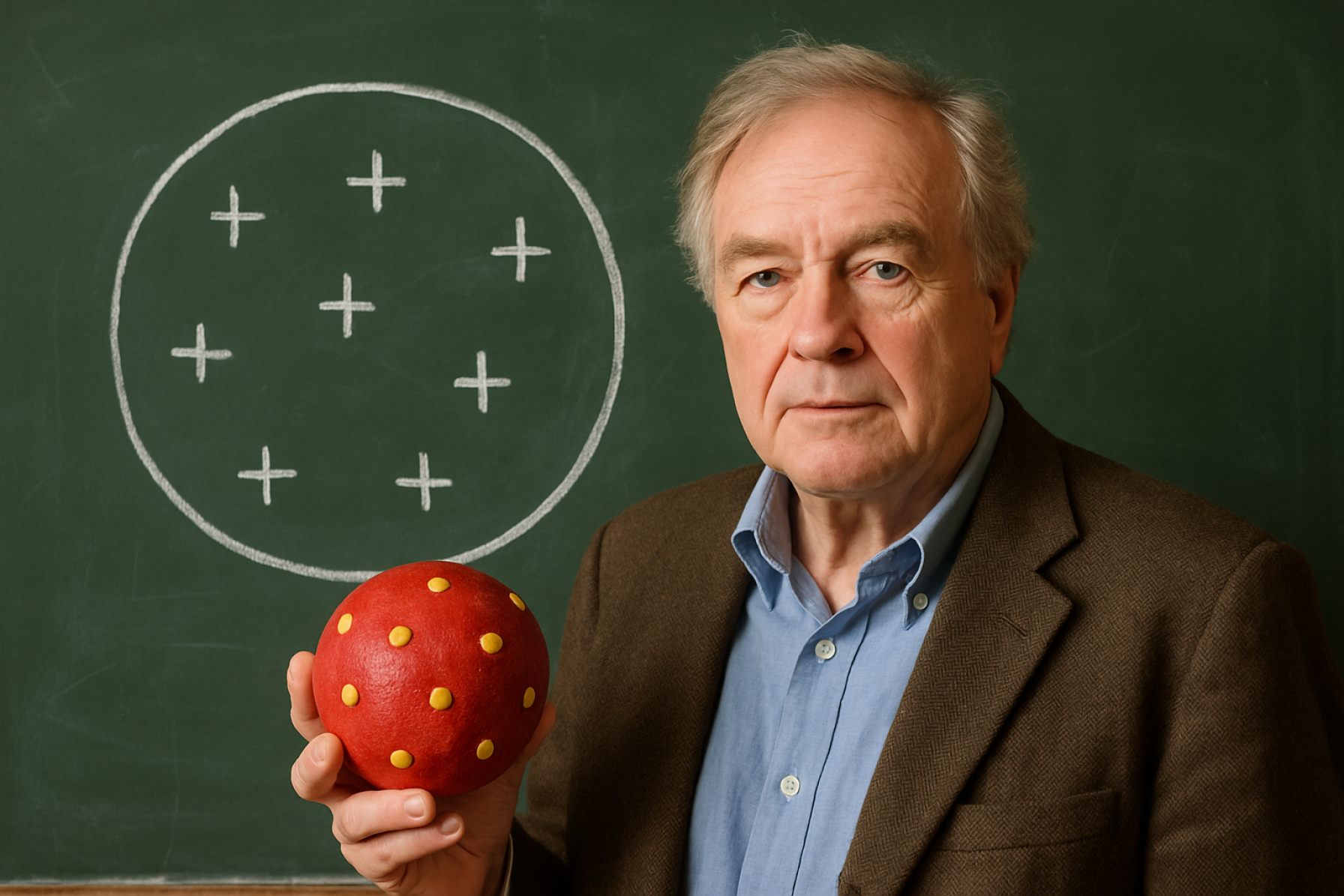
The Plumpudding Model, proposed by J.J. Thomson in 1904, marked a pivotal moment in the development of atomic theory. Prior to this model, the atom was largely considered indivisible, following John Dalton’s solid-sphere concept. However, Thomson’s discovery of the electron in 1897 fundamentally challenged this view, suggesting that atoms were, in fact, composed of smaller subatomic particles. In response, Thomson envisioned the atom as a positively charged “pudding” in which negatively charged electrons—akin to “plums”—were embedded, distributed throughout the atom to maintain electrical neutrality. This model was the first to incorporate the existence of internal structure within the atom, moving away from the idea of atoms as featureless spheres Royal Society of Chemistry.
The Plumpudding Model was significant not only for its novel depiction of atomic structure but also for its influence on subsequent scientific inquiry. It provided a framework for understanding how atoms could emit and absorb energy, and it spurred further experimental investigations into atomic structure. Although the model was eventually superseded by Ernest Rutherford’s nuclear model following the gold foil experiment, it remains a landmark in the history of science. The Plumpudding Model’s introduction signaled the beginning of modern atomic physics, highlighting the atom’s complexity and setting the stage for future discoveries Science History Institute.
J.J. Thomson and the Discovery of the Electron
J.J. Thomson’s discovery of the electron in 1897 fundamentally altered the scientific understanding of atomic structure and directly led to the formulation of the Plumpudding Model. Through his experiments with cathode rays, Thomson demonstrated that atoms contained small, negatively charged particles—later named electrons—contradicting the prevailing notion that atoms were indivisible and structureless. This breakthrough, recognized by The Nobel Prize, necessitated a new atomic model to account for the presence of these subatomic particles.
In response, Thomson proposed the Plumpudding Model in 1904. He envisioned the atom as a positively charged “pudding” in which negatively charged electrons (the “plums”) were embedded, distributed throughout the atom to balance the overall charge. This model was a significant departure from earlier atomic theories, such as John Dalton’s solid, indivisible sphere, and it provided a framework for understanding atomic neutrality and the existence of internal structure within atoms. The Plumpudding Model was widely accepted for a time and influenced subsequent research, including the experiments of Ernest Rutherford, which would later challenge and refine atomic theory.
Thomson’s work not only introduced the concept of subatomic particles but also set the stage for the rapid evolution of atomic models in the early 20th century. His contributions are documented by institutions such as the Royal Society of Chemistry and remain foundational in the history of atomic physics.
Core Concepts: Structure and Features of the Plumpudding Model
The Plumpudding Model, proposed by J.J. Thomson in 1904, was an early attempt to describe the internal structure of the atom following his discovery of the electron. In this model, the atom is envisioned as a positively charged “pudding” or sphere, within which negatively charged electrons (the “plums”) are embedded. The positive charge is thought to be spread uniformly throughout the atom, balancing the negative charge of the electrons to ensure overall electrical neutrality. This arrangement was intended to explain both the atom’s stability and the observed behavior of electrons in cathode ray experiments.
A key feature of the Plumpudding Model is the lack of a central nucleus; instead, the positive charge is diffuse and not concentrated in any specific region. Electrons are distributed throughout the atom, but their exact positions are not fixed—they are free to move within the positive matrix. This model also implied that the mass of the atom was distributed more or less evenly, with electrons contributing only a small fraction due to their much lower mass compared to the positive “pudding.”
While the Plumpudding Model was soon superseded by the nuclear model following Ernest Rutherford’s gold foil experiment, it was a crucial step in the development of atomic theory. It introduced the concept of subatomic structure and provided a framework for understanding atomic neutrality and the existence of electrons within atoms. For more details, see Royal Society of Chemistry and Encyclopaedia Britannica.
Scientific Impact: How the Model Challenged Previous Atomic Theories
The Plumpudding Model, proposed by J.J. Thomson in 1904, marked a significant departure from earlier atomic theories, particularly John Dalton’s solid, indivisible sphere model. Prior to Thomson’s work, atoms were considered the smallest, unbreakable units of matter, with no internal structure. The discovery of the electron by Thomson in 1897, however, necessitated a new conceptualization of atomic structure. The Plumpudding Model posited that atoms consisted of a diffuse, positively charged “pudding” in which negatively charged electrons (“plums”) were embedded. This was the first model to suggest that atoms were divisible and contained subatomic particles, fundamentally challenging Dalton’s atomic theory and the notion of atomic indivisibility Royal Society of Chemistry.
The model’s scientific impact was profound. It provided a framework for understanding the existence and behavior of electrons within atoms, prompting further experimental and theoretical investigations. The Plumpudding Model also set the stage for Ernest Rutherford’s gold foil experiment, which ultimately disproved the model but led to the development of the nuclear model of the atom. By introducing the concept of internal atomic structure, Thomson’s model catalyzed a paradigm shift in atomic physics, encouraging scientists to probe deeper into the nature of matter and paving the way for quantum theory and modern atomic models Nobel Prize.
Experimental Evidence: Support and Criticisms
The Plum Pudding Model, proposed by J.J. Thomson in 1904, was initially supported by experimental evidence from cathode ray tube experiments, which demonstrated the existence of negatively charged particles—later named electrons—within the atom. Thomson’s model depicted the atom as a positively charged “pudding” with embedded electrons, akin to plums in a dessert. This arrangement explained the atom’s overall electrical neutrality and accounted for the observed behavior of cathode rays, as detailed by Royal Society of Chemistry.
However, the model soon faced significant criticism following the results of the gold foil experiment conducted by Ernest Rutherford and his colleagues in 1909. In this experiment, alpha particles were directed at a thin sheet of gold foil, and while most passed through, a small fraction were deflected at large angles. This observation was inconsistent with the Plum Pudding Model, which predicted only minor deflections due to the diffuse positive charge. The unexpected results suggested the presence of a dense, positively charged nucleus at the atom’s center, leading to the development of the nuclear model of the atom. The experiment’s findings are comprehensively discussed by the Nobel Prize Organization.
Thus, while the Plum Pudding Model was a crucial step in atomic theory, its inability to explain the results of the gold foil experiment ultimately led to its replacement, highlighting the importance of experimental evidence in shaping scientific understanding.
The Fall of the Plumpudding Model: Rutherford’s Gold Foil Experiment
The downfall of the Plumpudding Model, proposed by J.J. Thomson in 1904, was precipitated by the groundbreaking gold foil experiment conducted by Ernest Rutherford and his colleagues in 1909. The Plumpudding Model envisioned the atom as a diffuse cloud of positive charge with negatively charged electrons embedded within, akin to raisins in a pudding. This model predicted that alpha particles, when directed at a thin sheet of gold foil, would pass through with minimal deflection, as the positive charge was thought to be spread out evenly throughout the atom.
However, Rutherford’s experiment revealed a startling result: while most alpha particles did pass through the foil, a small fraction were deflected at large angles, and some even rebounded directly backward. This observation was incompatible with the Plumpudding Model, as such significant deflections could only occur if the positive charge—and most of the atom’s mass—was concentrated in a tiny, dense region. Rutherford interpreted these results as evidence for a new atomic structure: the nucleus, a compact core containing all the positive charge and most of the mass, with electrons orbiting around it.
The gold foil experiment thus marked a pivotal moment in atomic theory, leading to the abandonment of the Plumpudding Model and the development of the nuclear model of the atom. This transformation in understanding is widely regarded as one of the most significant advances in early twentieth-century physics, as documented by the Royal Society of Chemistry and the Nobel Prize Organization.
Legacy and Influence on Modern Atomic Theory
The legacy of the Plumpudding Model, proposed by J.J. Thomson in 1904, is significant in the evolution of atomic theory, despite its eventual replacement by more accurate models. This model was the first to incorporate the existence of subatomic particles—specifically, electrons—within the atom, challenging the long-held notion of indivisible atoms from Dalton’s theory. By suggesting that negatively charged electrons were embedded in a positively charged “pudding,” Thomson’s model provided a conceptual framework for understanding atomic structure beyond the indivisible sphere, paving the way for further experimental inquiry Royal Society of Chemistry.
The Plumpudding Model’s most profound influence was its role as a stepping stone to the nuclear model of the atom. Ernest Rutherford’s gold foil experiment, which demonstrated that atoms have a small, dense, positively charged nucleus, was designed specifically to test the predictions of Thomson’s model. The experimental results, which showed that some alpha particles were deflected at large angles, could not be explained by the Plumpudding Model, leading to its eventual abandonment in favor of the nuclear model Nobel Prize.
Despite its shortcomings, the Plumpudding Model’s introduction of internal atomic structure and subatomic particles was a critical milestone. It stimulated a wave of research that ultimately led to the quantum mechanical model of the atom, influencing generations of physicists and chemists in their understanding of atomic and subatomic phenomena American Physical Society.
Conclusion: Lessons from the Plumpudding Model
The Plumpudding Model, though ultimately superseded, offers enduring lessons about the scientific process and the evolution of atomic theory. Proposed by J.J. Thomson in 1904, this model represented a significant step forward by incorporating the newly discovered electron into the structure of the atom. Its eventual replacement by the nuclear model, following the results of the gold foil experiment, underscores the importance of experimental evidence in shaping and refining scientific understanding. The Plumpudding Model illustrates how scientific models are provisional, serving as frameworks that are continually tested and revised in light of new data. This iterative process is central to scientific progress, as seen when Ernest Rutherford’s experiments revealed the existence of a dense atomic nucleus, prompting a paradigm shift in atomic theory Royal Society of Chemistry. Furthermore, the model’s limitations highlight the necessity of critical scrutiny and the willingness to abandon or modify theories that no longer align with empirical observations. In retrospect, the Plumpudding Model’s legacy lies not in its accuracy, but in its role as a catalyst for further inquiry and discovery. It exemplifies how even incorrect models can stimulate productive debate and experimentation, ultimately leading to a deeper and more accurate understanding of the natural world American Physical Society.